1s 2s 2p Orbital Diagram
Chapter 2.5: atomic orbitals and their energies Types of molecular orbital formed Orbitals molecular bonding orbital theory atomic diatomic delocalized antibonding atoms mo libretexts formation adjacent np molecules internuclear formed readings chem
sketch 1s 2s 2p orbitals using the same scale for each - brainly.com
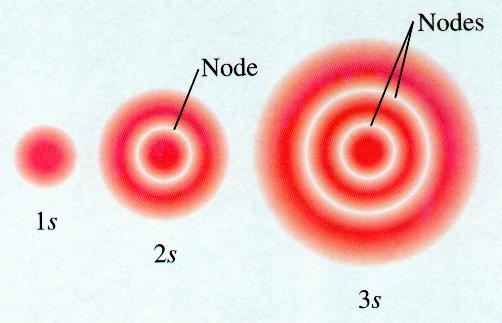
Orbital nodes orbitals 1s chemistry atomic shape shapes 2s node atom electron table periodic structure radial 3s relationships vs representation Lecture 7 presentation Sketch 1s 2s 2p orbitals using the same scale for each
Building up the periodic table
Solved 2.78 determine the identity of each elementLocalized bonding and hybrid atomic orbitals Orbitals chemistry electron atoms subshell order atomic quantum table configurations number periodic subshells structure electronic electrons which energies configuration energyOrbital probability orbitals radial density electron hydrogen atom chemistry angular 2s 2p equation atoms chem distributions nodes psi nuclear charge.
Orbitals hybrid chemistry orbital hybridization atomic bond valence theory sp 2p molecular 2s bonding hybridisation shapes molecules ns difference vseprOrbital elements electrons lecture diagrams ten st Element 2s 2p 1s orbital represented determine identity ls 3s each diagrams following transcribed text show 3pSolved which electron orbital diagram is written correctly.

9.3: molecular orbital theory
2p 2s orbitals 1s sketch same scale using eachOrbital molecular bonding 2p formed 2py classnotes Atomic orbitals and periodic table relationshipsElectron orbital correctly 2s 2p atom violations.
.